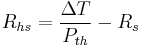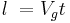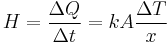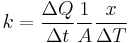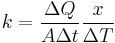Thermal conductivity
In physics, thermal conductivity, 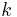 , is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction.
, is the property of a material's ability to conduct heat. It appears primarily in Fourier's Law for heat conduction.
Heat transfer across materials of high thermal conductivity occurs at a higher rate than across materials of low thermal conductivity. Correspondingly materials of high thermal conductivity are widely used in heat sink applications and materials of low thermal conductivity are used as thermal insulation. Thermal conductivity of materials is temperature dependent. The reciprocal of thermal conductivity is thermal resistivity.
Contents |
Units of thermal conductivity
In the International System of Units (SI), thermal conductivity is measured in watts per meter kelvin (W/(m·K)).
In the imperial system of measurement thermal conductivity is measured in Btu/(hr·ft⋅F) where 1 Btu/(hr·ft⋅F) = 1.730735 W/(m·K). [Perry's Chemical Engineers' Handbook, 7th Edition, Table 1-4]
Other units which are closely related to the thermal conductivity are in common use in the construction and textile industries. The construction industry makes use of units such as the R-Value (resistance value) and the U-Value (thermal transmittance). Although related to the thermal conductivity of a product R and U-values are dependent on the thickness of a product.
Likewise the textile industry has several units including the Tog and the Clo which express thermal resistance of a material in a way analogous to the R-values used in the construction industry.
Note: R-Values and U-Values quoted in the US (based on the imperial units of measurement) do not correspond with and are not compatible with those used in Europe (based on the SI units of measurement).
Measurement
There are a number of ways to measure thermal conductivity. Each of these is suitable for a limited range of materials, depending on the thermal properties and the medium temperature. There is a distinction between steady-state and transient techniques.
In general, steady-state techniques are useful when the temperature of the material does not change with time. This makes the signal analysis straightforward (steady state implies constant signals). The disadvantage is that a well-engineered experimental setup is usually needed. The Divided Bar (various types) is the most common device used for consolidated rock samples.
The transient techniques perform a measurement during the process of heating up. Their advantage is quicker measurements. Transient methods are usually carried out by needle probes. A method described by Angstrom involves rapidly cycling the temperature from hot to cold and back and measuring the temperature change as the heat propagates along a thin strip of material in a vacuum.
Definitions
The reciprocal of thermal conductivity is thermal resistivity, usually measured in kelvin-meters per watt (K·m·W−1). When dealing with a known amount of material, its thermal conductance and the reciprocal property, thermal resistance, can be described. Unfortunately, there are differing definitions for these terms.
Conductance
For general scientific use, thermal conductance is the quantity of heat that passes in unit time through a plate of particular area and thickness when its opposite faces differ in temperature by one kelvin. For a plate of thermal conductivity k, area A and thickness L this is kA/L, measured in W·K−1 (equivalent to: W/°C). Thermal conductivity and conductance are analogous to electrical conductivity (A·m−1·V−1) and electrical conductance (A·V−1).
There is also a measure known as heat transfer coefficient: the quantity of heat that passes in unit time through unit area of a plate of particular thickness when its opposite faces differ in temperature by one kelvin. The reciprocal is thermal insulance. In summary:
- thermal conductance = kA/L, measured in W·K−1
- thermal resistance = L /(kA), measured in K·W−1 (equivalent to: °C/W)
- heat transfer coefficient = k/L, measured in W·K−1·m−2
- thermal insulance = L /k, measured in K·m²·W−1.
The heat transfer coefficient is also known as thermal admittance
Resistance
It is a thermal-property of a material to resist the flow of heat.
It is a resistance offered by a material(a metal in general and a heat sink material in particular) to the conduction or flow of heat through it.
Lesser the Thermal Resistance better will be the heat conduction and vice versa.
When thermal resistances occur in series, they are additive. So when heat flows through two components each with a resistance of 1 °C/W, the total resistance is 2 °C/W.
A common engineering design problem involves the selection of an appropriate sized heat sink for a given heat source. Working in units of thermal resistance greatly simplifies the design calculation. The following formula can be used to estimate the performance:
where:
- Rhs is the maximum thermal resistance of the heat sink to ambient, in °C/W (equivalent to K/W)
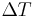 is the temperature difference (temperature drop), in °C
is the temperature difference (temperature drop), in °C- Pth is the thermal power (heat flow), in watts
- Rs is the thermal resistance of the heat source, in °C/W
For example, if a component produces 100 W of heat, and has a thermal resistance of 0.5 °C/W, what is the maximum thermal resistance of the heat sink? Suppose the maximum temperature is 125 °C, and the ambient temperature is 25 °C; then the 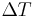 is 100 °C. The heat sink's thermal resistance to ambient must then be 0.5 °C/W or less.
is 100 °C. The heat sink's thermal resistance to ambient must then be 0.5 °C/W or less.
Transmittance
A third term, thermal transmittance, incorporates the thermal conductance of a structure along with heat transfer due to convection and radiation. It is measured in the same units as thermal conductance and is sometimes known as the composite thermal conductance. The term U-value is another synonym.
Influencing factors
Temperature
The effect of temperature on thermal conductivity is different for metals and nonmetals. In metals conductivity is primarily due to free electrons. Following Wiedemann–Franz law thermal conductivity of metals is approximately proportional to the absolute temperature (in Kelvin) times electrical conductivity. In pure metals the electrical resistivity often increases proportional to temperature and thus thermal conductivity stays approximately constant. In alloys the change in electrical conductivity is usually smaller and thus thermal conductivity increases with temperature, often proportional to temperature.
On the other hand conductivity in nonmetals is mainly due to lattice vibrations (phonons). Except for hight quality crystals at low temperatures, the phonon mean free path of phonons is not reduced significantly at higher temperatures. Thus the thermal conductivity of nonmetals is approximately constant at not to low temperatures. At low temperatures well below Debye-temperature thermal conductivity decreases just like the heat capacity does.
Material phase
When a material undergoes a phase change from solid to liquid or from liquid to gas the thermal conductivity may change. An example of this would be the change in thermal conductivity that occurs when ice (thermal conductivity of 2.18 W/(m·K) at 0 °C) melts into liquid water (thermal conductivity of 0.58 W/(m·K) at 0 °C).
Material structure
Pure crystalline substances can exhibit different thermal conductivities along different crystal axes, due to differences in phonon coupling along a given crystal axis. Sapphire is a notable example of variable thermal conductivity based on orientation and temperature, with 35 W/(m·K) along the c-axis and 32 W/(m·K) along the a-axis.[1]
Electrical conductivity
In metals, thermal conductivity approximately tracks electrical conductivity according to the Wiedemann-Franz law, as freely moving valence electrons transfer not only electric current but also heat energy. However, the general correlation between electrical and thermal conductance does not hold for other materials, due to the increased importance of phonon carriers for heat in non-metals. As shown in the table below, highly electrically conductive silver is less thermally conductive than diamond, which is an electrical insulator.
Convection
Air and other gases are generally good insulators, in the absence of convection. Therefore, many insulating materials function simply by having a large number of gas-filled pockets which prevent large-scale convection. Examples of these include expanded and extruded polystyrene (popularly referred to as "styrofoam") and silica aerogel. Natural, biological insulators such as fur and feathers achieve similar effects by dramatically inhibiting convection of air or water near an animal's skin.
Light gases, such as hydrogen and helium typically have high thermal conductivity. Dense gases such as xenon and dichlorodifluoromethane have low thermal conductivity. An exception, sulfur hexafluoride, a dense gas, has a relatively high thermal conductivity due to its high heat capacity. Argon, a gas denser than air, is often used in insulated glazing (double paned windows) to improve their insulation characteristics.
Experimental values
Thermal conductivity is important in building insulation and related fields. However, materials used in such trades are rarely subjected to chemical purity standards. Several construction materials' k values are listed below. These should be considered approximate due to the uncertainties related to material definitions. In the opposite end of the spectrum, solutions for computer cooling usually use high thermal capacity materials such as silver, copper and aluminium, to cool down specific components.
The following table is meant as a small sample of data to illustrate the thermal conductivity of various types of substances. For more complete listings of measured k-values, see the references.
This is a list of approximate values of thermal conductivity, k, for some common materials. Please consult the list of thermal conductivities for more accurate values, references and detailed information.
| Material | Thermal conductivity [W/(m·K)] |
|---|---|
| Silica Aerogel | 0.004 - 0.04 |
| Air | 0.025 |
| Wood | 0.04 - 0.4 |
| Hollow Fill Fibre Insulation | 0.042 |
| Alcohols and oils | 0.1 - 0.21 |
| Polypropylene | 0.25 [2] |
| Mineral oil | 0.138 |
| Rubber | 0.16 |
| LPG | 0.23 - 0.26 |
| Cement, Portland | 0.29 |
| Epoxy (silica-filled) | 0.30 |
| Epoxy (unfilled) | 0.12 - 0.177 [3][4] |
| Water (liquid) | 0.6 |
| Thermal grease | 0.7 - 3 |
| Thermal epoxy | 1 - 7 |
| Glass | 1.1 |
| Soil | 1.5 |
| Concrete, stone | 1.7 |
| Ice | 2 |
| Sandstone | 2.4 |
| Mercury | 8.3 |
| Stainless steel | 12.11 ~ 45.0 |
| Lead | 35.3 |
| Aluminium | 237 (pure) 120—180 (alloys) |
| Gold | 318 |
| Copper | 401 |
| Silver | 429 |
| Diamond | 900 - 2320 |
| Graphene | (4840±440) - (5300±480) |
Physical origins
Heat flux is exceedingly difficult to control and isolate in a laboratory setting. Thus at the atomic level, there are no simple, correct expressions for thermal conductivity. Atomically, the thermal conductivity of a system is determined by how atoms composing the system interact. There are two different approaches for calculating the thermal conductivity of a system.
- The first approach employs the Green-Kubo relations. Although this employs analytic expressions which in principle can be solved, calculating the thermal conductivity of a dense fluid or solid using this relation requires the use of molecular dynamics computer simulation.
- The second approach is based upon the relaxation time approach. Due to the anharmonicity within the crystal potential, the phonons in the system are known to scatter. There are three main mechanisms for scattering:
- Boundary scattering, a phonon hitting the boundary of a system;
- Mass defect scattering, a phonon hitting an impurity within the system and scattering;
- Phonon-phonon scattering, a phonon breaking into two lower energy phonons or a phonon colliding with another phonon and merging into one higher energy phonon.
Lattice waves
Heat transport in both glassy and crystalline dielectric solids occurs through elastic vibrations of the lattice (phonons). This transport is limited by elastic scattering of acoustic phonons by lattice defects. These predictions were confirmed by the experiments of Chang and Jones on commercial glasses and glass ceramics, where mean free paths were limited by "internal boundary scattering" to length scales of 10−2 cm to 10−3 cm. [5][6]
The phonon mean free path has been associated directly with the effective relaxation length for processes without directional correlation. Thus, if Vg is the group velocity of a phonon wave packet, then the relaxation length 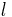 is defined as:
is defined as:
where t is the characteristic relaxation time. Since longitudinal waves have a much greater phase velocity than transverse waves, Vlong is much greater than Vtrans, and the relaxation length or mean free path of longitudinal phonons will be much greater. Thus, thermal conductivity will be largely determined by the speed of longitudinal phonons. [5][7]
Regarding the dependence of wave velocity on wavelength or frequency (dispersion), low-frequency phonons of long wavelength will be limited in relaxation length by elastic Rayleigh scattering. This type of light scattering form small particles is proportional to the fourth power of the frequency. For higher frequencies, the power of the frequency will decrease until at highest frequencies scattering is almost frequency independent. Similar arguments were subsequently generalized to many glass forming substances using Brillouin scattering. [8][9] [10] [11]
Phonons in the acoustical branch dominate the phonon heat conduction as they have greater energy dispersion and therefore a greater distribution of phonon velocities. Additional optical modes could also be caused by the presence of internal structure (i.e., charge or mass) at a lattice point; it is implied that the group velocity of these modes is low and therefore their contribution to the lattice thermal conductivity λL (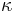 L) is small. [12]
L) is small. [12]
Each phonon mode can be split into one longitudinal and two transverse polarization branches. By extrapolating the phenomenology of lattice points to the unit cells it is seen that the total number of degrees of freedom is 3pq when p is the number of primitive cells with q atoms/unit cell. From these only 3p are associated with the acoustic modes, the remaining 3p(q-1) are accommodated through the optical branches. This implies that structures with larger p and q contain a greater number of optical modes and a reduced λL.
From these ideas, it can be concluded that increasing crystal complexity, which is described by a complexity factor CF (defined as the number of atoms/primitive unit cell), decreases λL. Micheline Roufosse and P.G. Klemens derived the exact proportionality in their article Thermal Conductivity of Complex Dielectric Crystals at Phys. Rev. B 7, 5379–5386 (1973). This was done by assuming that the relaxation time τ decreases with increasing number of atoms in the unit cell and ten scaling the parameters of the expression for thermal conductivity in high temperatures accordingly. [12]
Describing of anharmonic effects is complicated because exact treatment as in the harmonic case is not possible and phonons are no longer exact eigensolutions to the equations of motion. Even if the state of motion of the crystal could be described with a plane wave at a particular time, its accuracy would deteriorate progressively with time. Time development would have to be described by introducing a spectrum of other phonons, which is known as the phonon decay. The two most important anharmonic effects are the thermal expansion and the phonon thermal conductivity.
Only when the phonon number ‹n› deviates from the equilibrium value ‹n›0, can a thermal current arise as stated in following expression
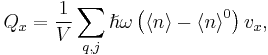
where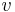 is the energy transport velocity of phonons. Only two mechanisms exist that can cause time variation of ‹n› in a particular region. The number of phonons that diffuse into the region from neighboring regions differs from those that diffuse out, or phonons decay inside the same region into other phonons. A special form of the Boltzmann equation
is the energy transport velocity of phonons. Only two mechanisms exist that can cause time variation of ‹n› in a particular region. The number of phonons that diffuse into the region from neighboring regions differs from those that diffuse out, or phonons decay inside the same region into other phonons. A special form of the Boltzmann equation
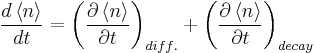
states this. When steady state conditions are assumed the total time derivate of phonon number is zero, because the temperature is constant in time and therefore the phonon number stays also constant. Time variation due to phonon decay is described with a relaxation time (τ) approximation
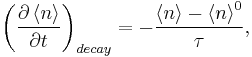
which states that the more the phonon number deviates from its equilibrium value, the more its time variation increases. At steady state conditions and local thermal equilibrium are assumed we get the following equation
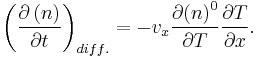
Using the relaxation time approximation for the Boltzmann equation and assuming steady-state conditions, the phonon thermal conductivity λL can be determined. The temperature dependence for λL originates from the variety of processes, whose significance for λL depends on the temperature range of interest. Mean free path is one factor that determines the temperature dependence for λL, as stated in the following equation
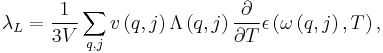
where Λ is the mean free path for phonon. This equation is a result of combining the four previous equations with each other and knowing that 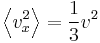 for cubic or isotropic systems and
for cubic or isotropic systems and 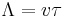 . [13]
. [13]
At low temperatures (<10 K) the anharmonic interaction does not influence the mean free path and therefore, the thermal resistivity is determined only from processes for which q-conservation does not hold. These processes include the scattering of phonons by crystal defects, or the scattering from the surface of the crystal in case of high quality single crystal. Therefore, thermal conductance depends on the external dimensions of the crystal and the quality of the surface. Thus, temperature dependence of λL is determined by the specific heat and is therefore proportional to T3. [13]
Phonon quasimomentum is defined as ℏq and differs from normal momentum due to the fact that it is only defined within an arbitrary reciprocal lattice vector. At higher temperatures (10 K<T <Θ), the conservation of energy 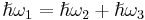 and quasimomentum
and quasimomentum 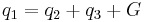 , where q1 is wave vector of the incident phonon and q2, q3 are wave vectors of the resultant phonons, may also involve a reciprocal lattice vector G complicating the energy transport process. These processes can also reverse the direction of energy transport.
, where q1 is wave vector of the incident phonon and q2, q3 are wave vectors of the resultant phonons, may also involve a reciprocal lattice vector G complicating the energy transport process. These processes can also reverse the direction of energy transport.
Therefore, these processes are also known as Umklapp (U) processes and can only occur when phonons with sufficiently large q-vectors are excited, because unless the sum of q2 and q3 points outside of the Brillouin zone the momentum is conserved and the process is normal scattering (N-process). The probability of a phonon to have energy E is given by the Boltzmann distribution 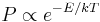 . To U-process to occur the decaying phonon to have a wave vector q1 that is roughly half of the diameter of the Brillouin zone, because otherwise quasimomentum would not be conserved.
. To U-process to occur the decaying phonon to have a wave vector q1 that is roughly half of the diameter of the Brillouin zone, because otherwise quasimomentum would not be conserved.
Therefore, these phonons have to possess energy of 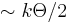 , which is a significant fraction of Debye energy that is needed to generate new phonons. The probability for this is proportional to
, which is a significant fraction of Debye energy that is needed to generate new phonons. The probability for this is proportional to 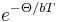 , with
, with 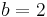 . Temperature dependence of the mean free path has an exponential form
. Temperature dependence of the mean free path has an exponential form 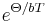 . The presence of the reciprocal lattice wave vector implies a net phonon backscattering and a resistance to phonon and thermal transport resulting finite λL[12], as it means that momentum is not conserved. Only momentum non-conserving processes can cause thermal resistance. [13]
. The presence of the reciprocal lattice wave vector implies a net phonon backscattering and a resistance to phonon and thermal transport resulting finite λL[12], as it means that momentum is not conserved. Only momentum non-conserving processes can cause thermal resistance. [13]
At high temperatures (T>Θ) the mean free path and therefore λL has a temperature dependence T-1, to which one arrives from formula 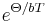 by making the following approximation
by making the following approximation 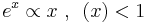 and writing
and writing 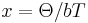 . This dependency is known as Eucken’s law and originates from the temperature dependency of the probability for the U-process to occur. [12][13]
. This dependency is known as Eucken’s law and originates from the temperature dependency of the probability for the U-process to occur. [12][13]
Thermal conductivity is usually described by the Boltzmann equation with the relaxation time approximation in which phonon scattering is a limiting factor. Another approach is to use analytic models or molecular dynamics or Monte Carlo based methods to describe thermal conductivity in solids.
Short wavelength phonons are strongly scattered by impurity atoms if an alloyed phase is present, but mid and long wavelength phonons are less affected. Mid and long wavelength phonons carry significant fraction of heat, so to further reduce lattice thermal conductivity one has to introduce structures to scatter these phonons. This is achieved by introducing interface scattering mechanism, which requires structures whose characteristic length is longer than that of impurity atom. Some possible ways to realize these interfaces are nanocomposites and embedded nanoparticles/structures. [14]
Electronic thermal conductivity
Hot electrons from higher energy states carry more thermal energy than cold electrons, while electrical conductivity is rather insensitive to the energy distribution of carriers because the amount of charge that electrons carry, does not depend on their energy. This is a physical reason for the greater sensitivity of electronic thermal conductivity to energy dependence of density of states and relaxation time, respectively. [12]
Mahan and Sofo have showed in their article The best thermoelectric (PNAS 1996 93 (15) 7436-7439) that materials with a certain electron structure have reduced electron thermal conductivity. Based on their analysis one can demonstrate that if the electron density of states in the material is close to the delta-function, the electronic thermal conductivity drops to zero. By taking the following equation 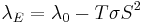 , where λ0 is the electronic thermal conductivity when the electrochemical potential gradient inside the sample is zero, as a starting point. As next step the transport coefficients are written as following
, where λ0 is the electronic thermal conductivity when the electrochemical potential gradient inside the sample is zero, as a starting point. As next step the transport coefficients are written as following 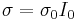 ,
, 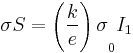 ,
, 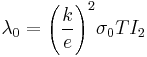 , where
, where 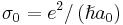 , with a0 as the Bohr’s radius. The dimensionless integrals In are defined as
, with a0 as the Bohr’s radius. The dimensionless integrals In are defined as 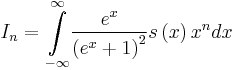 , where s(x) is the dimensionless transport distribution function. The integrals In are the moments of the function
, where s(x) is the dimensionless transport distribution function. The integrals In are the moments of the function 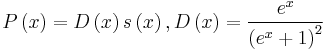 , x is the energy of carriers. By substituting the previous formulas for the transport coefficient to the equation for λE we get the following equation
, x is the energy of carriers. By substituting the previous formulas for the transport coefficient to the equation for λE we get the following equation 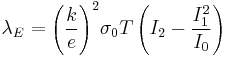 . From the previous equation we see that λE to be zero the bracketed term containing In terms have to be zero. Now if we assume that
. From the previous equation we see that λE to be zero the bracketed term containing In terms have to be zero. Now if we assume that 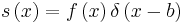 , where δ is the Dirac delta function, In terms get the following expressions
, where δ is the Dirac delta function, In terms get the following expressions 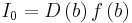 ,
, 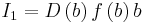 ,
, 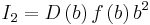 . By substituting these expressions to the equation for λE, we see that it goes to zero. Therefore, P(x) has to be delta function. [14]
. By substituting these expressions to the equation for λE, we see that it goes to zero. Therefore, P(x) has to be delta function. [14]
Equations
First, we define heat conduction, H:
where 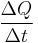 is the rate of heat flow, k is the thermal conductivity, A is the total cross sectional area of conducting surface, ΔT is temperature difference, and x is the thickness of conducting surface separating the two temperatures. Dimension of thermal conductivity = M1L1T−3K−1
is the rate of heat flow, k is the thermal conductivity, A is the total cross sectional area of conducting surface, ΔT is temperature difference, and x is the thickness of conducting surface separating the two temperatures. Dimension of thermal conductivity = M1L1T−3K−1
Rearranging the equation gives thermal conductivity:
(Note: 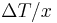 is the temperature gradient)
is the temperature gradient)
I.E. It is defined as the quantity of heat, ΔQ, transmitted during time Δt through a thickness x, in a direction normal to a surface of area A, per unit area of A, due to a temperature difference ΔT, under steady state conditions and when the heat transfer is dependent only on the temperature gradient.
Alternatively, it can be thought of as a flux of heat (energy per unit area per unit time) divided by a temperature gradient (temperature difference per unit length)
See also
References
- ^ http://www.almazoptics.com/sapphire.htm
- ^ Walter Michaeli, Extrusion Dies for Plastics and Rubber, 2nd Ed., Hanser Publishers, New York, 1992.
- ^ "3M Scotch-Weld DP125 datasheet". 3M. http://multimedia.3m.com/mws/mediawebserver?mwsId=SSSSSu7zK1fslxtUO8_Zm8fSev7qe17zHvTSevTSeSSSSSS--&fn=78690098666.pdf. Retrieved 21 April 2011.
- ^ "3M Scotch-Weld 270". 3M. http://multimedia.3m.com/mws/mediawebserver?mwsId=66666UuZjcFSLXTtnxfcOX46EVuQEcuZgVs6EVs6E666666--&fn=78690096777.PDF. Retrieved 21 April 2011.
- ^ a b P.G. Klemens (1951). "The Thermal Conductivity of Dielectric Solids at Low Temperatures". Proc. Roy. Soc. Lond. A 208 (1092): 108. Bibcode 1951RSPSA.208..108K. doi:10.1098/rspa.1951.0147.
- ^ G.K. Chan, R.E Jones (1962). "Low-Temperature Thermal Conductivity of Amorphous Solids". Phys. Rev. 126 (6): 2055. Bibcode 1962PhRv..126.2055C. doi:10.1103/PhysRev.126.2055.
- ^ I. Pomeranchuk (1941). "Thermal conductivity of the paramagnetic dielectrics at low temperatures". J. Phys.(USSR) 4: 357. ISSN 0368-3400.
- ^ R.C. Zeller, R.O. Pohl (1971). "Thermal Conductivity and Specific Heat of Non-crystalline Solids". Phys. Rev. B 4 (6): 2029. Bibcode 1971PhRvB...4.2029Z. doi:10.1103/PhysRevB.4.2029.
- ^ W.F. Love (1973). "Low-Temperature Thermal Brillouin Scattering in Fused Silica and Borosilicate Glass". Phys. Rev. Lett. 31 (13): 822. Bibcode 1973PhRvL..31..822L. doi:10.1103/PhysRevLett.31.822.
- ^ M.P. Zaitlin, M.C. Anderson (1975). "Phonon thermal transport in noncrystalline materials". Phys. Rev. B 12 (10): 4475. Bibcode 1975PhRvB..12.4475Z. doi:10.1103/PhysRevB.12.4475.
- ^ M.P. Zaitlin, L.M. Scherr, M.C. Anderson (1975). "Boundary scattering of phonons in noncrystalline materials". Phys. Rev. B 12 (10): 4487. Bibcode 1975PhRvB..12.4487Z. doi:10.1103/PhysRevB.12.4487.
- ^ a b c d e Paothep Pichanusakorn, Prabhakar Bandaru. Nanostructured thermoelectrics, Materials Science and Engineering: R: Reports, Volume 67, Issues 2-4, 29 January 2010, Pages 19-63, ISSN 0927-796X, DOI: 10.1016/j.mser.2009.10.001.
- ^ a b c d Ibach, Harald. ; Luth, Hans. Solid-state physics : an introduction to principles of materials science / Harald Ibach, Hans Luth. New York: Springer, 2009. -(Advanced texts in physics) ISBN 978-3-540-93803-3
- ^ a b A. J. Minnich, M. S. Dresselhaus, Z. F. Ren and G. Chen. Bulk nanostructured thermoelectric materials: current research and future prospects, Energy & Environmental Science, 2009, 2, 466-479, DOI: 10.1039/b822664b
Further reading
- Callister, William (2003). "Appendix B". Materials Science and Engineering - An Introduction. John Wiley & Sons, INC. pp. 757. ISBN 0-471-22471-5.
- Halliday, David; Resnick, Robert; & Walker, Jearl(1997). Fundamentals of Physics (5th ed.). John Wiley and Sons, INC., NY ISBN 0-471-10558-9.
- Srivastava G. P (1990), "The Physics of Phonons." Adam Hilger, IOP Publishing Ltd, Bristol.
- TM 5-852-6 AFR 88-19, Volume 6 (Army Corp of Engineers publication)
External links
- Table with the Thermal Conductivity of the Elements
- Calculation of the Thermal Conductivity of Glass Calculation of the Thermal Conductivity of Glass at Room Temperature from the Chemical Composition
- Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air
- Conversion of thermal conductivity values for many unit systems